Despite primary prevention strategies, heart disease is still the #1 cause of death in the United States. Every 25 seconds, an American will have a coronary event. Which means that – in the time since I started writing this post – someone has already experienced an event.
Let that sink in.
Which alsooooo means….that ACS ain’t going nowhere, unfortunately. So grab a snack and get ready to go through it.
To get a decent understanding of ACS, I decided to do a multi-post series. Today will focus on pathophysiology alone. I’m a strong believer that understanding pathophy is important to understanding treatments.
ACS, or acute coronary syndromes describes a range of conditions associated with a sudden reduction of blood flow to the heart.
This might be a little TMI for the purposes of this discussion, but not all ACS events are caused by the pathophys we will be discussing today. For example, there are things called type II myocardial infarctions that can cause a sudden reduction of blood flow to the heart from other things, like coronary vasospasms.

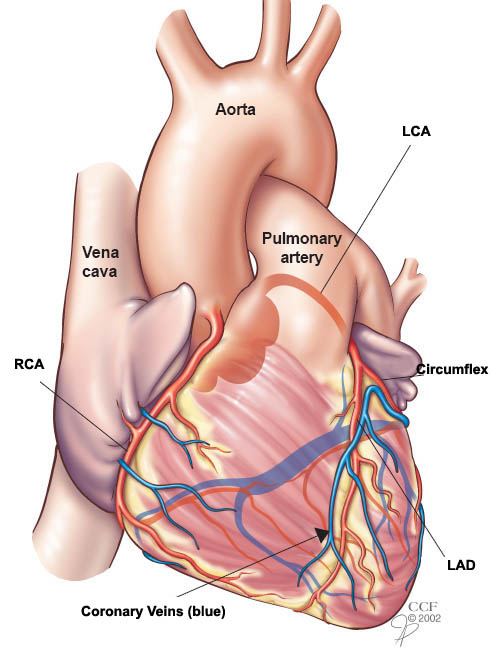
If you remember from the post on coronary anatomy, the heart, just like any organ, needs its own supply of oxygenated blood. Enter the coronary arteries. The coronary arteries branch off of the very beginning of the aorta and provide the heart with the blood flow and oxygen it needs to function.

Unfortunately, issues can happen with this blood flow supply, and a big driver causing issues with its blood supply is due to something called atherosclerosis. Atherosclerosis is the process of inflammation where lipid, cholesterol, calcium and other nasty stuff accumulate in the wall of the larger arteries – and in today’s case – in the coronary arteries.
Eventually, more and more fatty materials get deposited within this wall (mostly driven by the deposition of low-density lipoproteins or LDL – which is where statins will play a role later).

Your body recognizes this deposition of fatty materials as being somewhat “abnormal”, and so your body activates your immune system and sends macrophages to this site.
Macrophages are just specialized immune cells that (among other things) are meant to detect, phagocytose (aka eat up) and destroy bacteria and other harmful stuff in the body.
Once within that lipid layer in the vessel wall, the macrophages become known as foam cells.
However, even though they were sent there to fix the problem, THEY later die and accumulate in that lining of the vessel too…..
Nice try body, but maybe do better next time? Kthx.

Due to all this lipid/fatty deposition in the endothelial lining within your coronary arteries, your vessel wall ends up forming some scar tissue on its surface, which helps to keep that inner plaque in check.
This area of scar tissue above the lipid core is known as a fibrous cap.
However, all this scar tissue also makes the vessel wall tough and less elastic. Because of this loss in elasticity, patients with atherosclerosis end up getting hypertension since their vessels are less pliable.

Over time, this lipid layer becomes thicker and thicker and the space for the blood within the coronary vessel to pass through gets smaller and smaller. As this lipid layer bulge begins to protrude further into the coronary artery, you might start showing signs and symptoms of stable angina.
Angina is another term for chest pain. In patients with stable angina, under normal conditions, enough blood is able to squeeze through to oxygenate that heart tissue.
However, in times of physical activity or emotional stress when your heart starts pumping faster, the oxygen demand that the heart needs increases.
And, simply put – that little narrow hallway that you call a coronary artery is just too small to get enough blood through quickly enough to supply the heart.

I mean seriously ⬆️⬆️⬆️⬆️ look how tiny that passage is!!!!
And because the heart is not getting the supply of blood it needs to meet its demand, your heart undergoes some ischemia (aka inadequate blood supply) and you will experience chest pain, or angina.
However, with a couple minutes of rest and/or calming down, the demand/supply mismatch will even out and your heart will once again be able to get enough blood flow for its slower, more chilled out pace again. And your angina will go away.
That fibrous cap will live to hold that lipid layer in another day.
Until……………..
…
…
..
Suddenly, due to physical activity/emotional distress and sometimes, for no reason at all – that fibrous plaque just isn’t able to hold that lipid layer in anymore and it ruptures and busts open.

Now…in order to really get a good understanding of this next part, we have to revisit the process of CLOT FORMATION. I don’t know about you, but even I get flashbacks of memorizing the coagulation cascade in school.

But fear not, let’s talk about the basics now.

In school I remember talking about the coagulation cascade and learning about platelets. But, if you are as clueless as I was (and for the sake of my sanity – and ego – I’ll pretend that you are) I didn’t really grasp at the time of how these two pathways actually interact together to form a thrombus, or clot.
Now, I’m not going to go into TOO much detail for the sake of this post (and your sanity), but we could always discuss specifics at some other time.
Thrombus formation often starts due to damage of the vessel wall. Your vessels contain collagen and other good stuff that we’ll get into that they keep internalized within their vascular wall.
No injury – no problem – all that good stuff will stay within the vasculature wall.
However, with injury, collagen will be exposed to the bloodstream and so will proteins like von willebrand factor. VWF is a protein that lines the inside surface of blood vessels. However, if exposed to the bloodstream (like when there’s vessel damage), vWF helps to attract platelets to adhere to the walls of the blood vessel at the site of damage.
Platelets then undergo this whole activation cascade where they change shape to become even stickier and plug up that hole/damage. And voila! Platelet plug.

Now that we talked a little bit about platelets, let’s talk about the coagulation cascade side of things. It’s time to show you a *triggering* diagram of the coag cascade.


Before you get bogged down in all the details, let talk about the main purpose of the coagulation cascade.
The main purpose of the cascade is to make its final product – fibrin.
So what is fibrin? Fibrin is a tough protein substance that comes in long, fibrous (hence the name) chains. Fibrin acts to solidify the platelet plug and stabilize the clot. I like to think of fibrin as a heavy net that keeps the platelet plug in place.


Next, it’s time to look at what things can trigger the coagulation cascade (you might want to scroll up at check out the coag cascade diagram during this part).
The coag cascade is made up of two different pathways – the intrinsic pathway and the extrinsic pathway.
When I think about the intrinsic pathway, I think of your blood clotting due to being exposed to something foreign. The intrinsic pathway is started when factor XII is activated by certain negatively charged surfaces (for example glass, or other foreign materials).
When I think about the extrinsic pathway, I think of your blood clotting due to trauma and direct injury to the surface of the vessel wall. The extrinsic pathway is activated in the presence of something called tissue factor (TF) which is another protein present within the vessel wall.
When your vessel wall undergoes injury, this tissue factor is exposed to the bloodstream, the extrinsic pathway is triggered, and the process of creating fibrin will get started. And thus, that platelet plug should be solidified.
Which brings us back to the lipid plaque present in your endothelial wall being busted open in an acute coronary syndrome.

As this plaque busts open through the vessel wall, both platelet activation and the activation of the coagulation cascade will occur forming a clot at the side of the plaque rupture.
It’s actually the formation of this huge clot at this site occludes blood flow in the coronary artery.
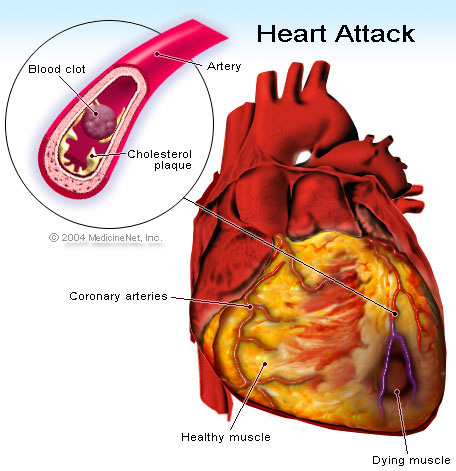
Understanding this pathophysiology is key to understanding the treatment of acute coronary syndromes.
With blood flow now being either partially or completely occluded by a thrombus in that coronary artery, any part of your heart tissue distal (further away) to that thrombus will no longer receive adequate blood flow/oxygen. And because of this, that heart tissue, the myocardium or muscle cells, will die.
The more amount of time that heart muscle is deprived of oxygen, the worse the damage and the higher the likelihood the damage will be irreversible.
With the heart muscle dying, patients may now develop acute systolic heart failure, which occurs when the heart muscle of the left ventricle is weakened or dead and is no longer able to generate adequate contraction to push blood out to the body.
Again, remember that the term systole refers to the part of the cardiac cycle where the heart undergoes contraction – so it should make sense that this type of contraction-related heart failure is known as systolic heart failure.
If patients are able to get their coronary artery reperfused in time and restore blood flow, this damage to the heart muscle may or may not be irreversible, depending on how long the ischemia happened.
Unfortunately, patients that have severe ischemic systolic heart failure are at high risk of developing ventricular arrhythmias (abnormal heart rhythm/conduction within the ventricles).

Because your heart and body is so dependent on the strength and contraction of your ventricles to propel blood to the body, anytime your ventricles beat too fast or quiver (fibrillate), blood flow to your body is either greatly or completely reduced (this is known in the hospital as a CODE BLUE, or cardiac arrest). Ventricular dysrhythmias such as ventricular tachycardia (VTACH) or ventricular fibrillation (VFIB) are examples of dysrhythmias that respond to shock.

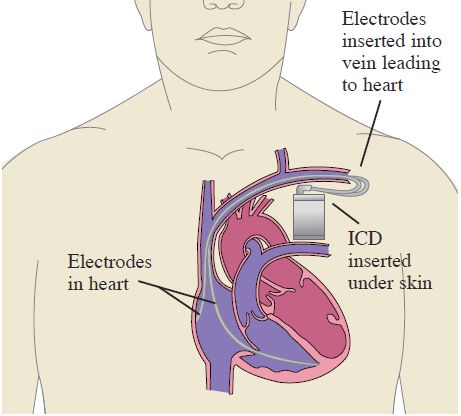
If your heart is able to recover some of its squeezing potential, it usually will do so within 40 days after your heart attack. Generally part of the treatment for these patients who have low squeezing potential and high risk of ventricular dysrhythmias involves implanting an ICD, or implantable cardioverter defibrillator.
An ICD is a device that is surgically implanted under the skin that will track your heart’s rhythm, detect dysrhythmias, and shock you to a normal rhythm if needed.
However, because this procedure is invasive and patients may still recover some of their squeezing power within 40 days after an event, patients will often get sent home with something known as a LifeVest.


A LifeVest is essentially an external ICD.
The only problem with the LifeVest is that it is super expensive (last time I checked, it was around $3,370/mo to lease 💸💸💸) and in order to work – the patient has to be wearing it. The LifeVest can’t be worn in water which means patients have to take it off during bathing or showering which leaves them exposed to cardiac arrest during these times.
Every other time – even while sleeping – the patient should have it on.
After the 40 days, the patient will undergo an echocardiogram which is a type of ultrasound that allows providers to see the heart’s structure, valves, and pumping power.
If the patient’s squeezing power hasn’t improved enough, it’s at this point that the patient will then undergo the surgical implantation of an ICD.

Stay tuned for future posts that will have an overview of diagnosis, acute medication therapy, reperfusion strategies and long-term/discharge medication therapy for ACS events.

That is a great tip especially to those fresh to the blogosphere. Brief but very precise information… Many thanks for sharing this one. A must read post!
LikeLike
There’s definately a lot to know about this issue. I really like all the points you’ve made.
LikeLike