Now that we talked about pathophys and diagnosis, let’s finally talk about meds and treatment (after all, as a clinical pharmacist, I’m the meds person on the team 🤓).
Today we are going to talk about meds that you should initiate ASAP.
I don’t know about you, but when I was in school I learned about MONA (M= morphine, O=oxygen, N=nitrates and A=antiplatelets/anticoag). It was definitely a helpful acronym at the time, but today I’m going to talk about why I don’t really love it.

I want to start off with arguably the most important medications you should be giving during ACS – antiplatelets and anticoagulation. All these other treatments we’re going to talk about later might help your patient feel better in the moment, but it’s not going to treat the underlying cause (I don’t care how much morphine you give your patient, it’s not going to solve that clot issue in your coronary artery).
Since you already read and understand the pathophys of ACS, you should already be on board for why we want to use antiplatelets and anticoagulants. We want to prevent this thrombus in the coronary artery from growing any bigger, and to do that, we are are going to target both the platelet cascade and the coagulation cascade to prevent that clot from getting any bigger.
Let’s start with the antiplatelets. Patients with ACS should get what we call dual antiplatelet therapy, which consists of a combination of aspirin and a P2Y12 inhibitor.

Aspirin
Invented in the 1800s, aspirin is still the backbone of therapy for ACS. Aspirin was discovered based on compounds in plants such as the willow tree.

Chemist Charles Gerdhardt created aspirin (aka acetylsalicyclic acid) for the first time by combining sodium salicylate with acetyl chloride. The name aspirin came from the prefix a(cetyl) + spir (from spireaea a plant genus where it acetylsalicylic was derived) + in (a common chemical suffix at the time).
Literally used for over a hundred years, it wasn’t until 1971 where John Vane figured out the mechanism of action of how aspirin works. Aspirin suppresses the production of prostaglandins and thromboxanes in the body by irreversibly inactivating an enzyme known as COX (cyclooxygenase) which is needed to synthesize prostaglandins and thromboxanes.
Prostaglandins are a group of lipid compounds that have a bunch of different effects in animals and are found in almost every tissue in humans. Different prostaglandins have different structural differences which make them have different effects in the body. Prostaglandins are involved in inflammation, regulating contraction of smooth muscle tissues, and prevent needless clot formation. Not as important in ACS, but prostaglandins are also involved in hypothalamic temperature regulation and transmission of pain to the brain, which is why aspirin can be effective for treating fever and pain.
Thromboxanes are other lipids that are involved in vasoconstriction and facilitates platelet aggregation. This is actually how thromboxane got its name (read: thrombus). In ACS when platelets become activated and sticky to form a plug, these platelets secrete a bunch of different stuff to aid in clot formation, and among these is thromboxane A2 (TXA2). Thromboxane A2 stimulates the activation of new platelets and increases platelet aggregation.

By irreversibly inhibiting the COX enzyme, aspirin prevents further prostaglandin and thromboxane synthesis, therefore decreasing platelet activation and inflammation.
It gets a little bit more tricky though – there’s actually more than 1 type of COX enzyme in your body. Aspirin works on both COX-1 and COX-2, which is why, besides our effects we want from aspirin, we also see some undesirable side effects (btw, we didn’t figure out that there were two of these guys until the 1990s).
COX-1 is present in most tissues in our bodies. In the gastrointestinal tract, COX-1 helps to maintain the normal lining of our stomach, intestines, etc and protects the stomach from our acidic and digestive juices. COX-1 is also the guy that makes the stuff that activates platelets, which is what we want to target in ACS.
COX-2 is primarily found at sites of inflammation and does not really have any effects on platelets. It doesn’t help protect the stomach. It mostly is involved in producing the prostaglandins that contribute to pain, fever, and inflammation.

So this leads us in a little conundrum. We want to block both COX-1 and COX-2, but unfortunately the unwanted effect here is that by blocking COX-1 we also can end up with side effects like gastrointestinal (GI) bleeding.
(Side note: though they’re not used in ACS, we actually have made COX-2 selective inhibitors (e.g. celecoxib) which is used to treat pain and inflammation, without getting the blood-thinning and GI bleeding effects we see with aspirin.
(side-side note: if anyone has a baby and needs a quick halloween costume idea, I vote baby aspirin. get a white onesie, slap a little printed bayer logo on them, and you’re done. I’ve always had this idea but don’t have a baby to do it with and my dogs don’t cooperate).
In ACS, aspirin should be given orally as a load (usually 325 mg)– chewed, followed by a maintenance dose of 81 mg (baby aspirin dosing) by mouth per day.
Given aspirin is one of our oldest agents, data supporting its use in ACS was published back in 1988 in the ISIS-2 trial. I cannot let you learn about aspirin without talking about how much of a badass move the authors of the ISIS-2 trial pulled on the reviewers of the Lancet. After submitting their paper for publication, reviewers said in order to publish, they wanted the authors to conduct and include a subgroup analysis. Subgroup analyses are basically a way to look at data based on certain variables to see what the treatment effect is within that specific group. For example, you could stratify data based on gender, age, etc and see if the effect is different or consistent in males versus women, for example.
Annoyed at the request, the authors decided to comply – but not before including a subgroup analysis based on astrological sign. Yep, you heard me.

And they actually found that Geminis and Libras were actually more likely to die with aspirin use. 🤷♀️🤷♀️🤷♀️

I love, love, love this trial to demonstrate to learners that 👏subgroup👏analysis👏is👏hypothesis👏generating👏only👏. In other words, you go fishing with your data enough, and you’re bound to find difference between groups by chance.
P2Y12 Inhibitors
Now let’s get into our other antiplatelet backbone – P2Y12 inhibitors.
What’s a P2Y12 thingy maboby?
The mechanism of action (MOA) of P2Y12 receptor antagonists are way more straightforward than aspirin’s.

The P2Y12 receptor is a receptor that is present on the surface of platelets and plays an important part in platelet aggregation. When activated by ADP, platelet aggregation is induced.
By creating drugs that can antagonize, or inhibit, this receptor, we can then reduce and prevent platelet aggregation.
Our oral P2Y12 inhibitors are clopidogrel (Plavix), ticagrelor (Brilinta) and prasugrel (Effient).
Believe it or not, aspirin was our main girl for years….in fact, our first P2Y12 inhibitor get approved until the end of 1997– and her name was clopidogrel (brand name Plavix). Thanks to the landmark CURE trial, we found that adding clopidogrel to aspirin in patients with UA/NSTEMI significantly reduced the composite endpoint of CV mortality, non-fatal MI or stroke with the tradeoff that it also increased the rate of major bleeding (3.7% versus 2.7%). Given the benefits outweighed the risks, the new standard of care became for ACS became dual antiplatelet therapy (DAPT) with aspirin and clopidogrel.
Clopidogrel is an oldie but goodie in terms of our P2Y12 inhibitor options, but she has her flaws.
Pros: Because she’s older, she’s affordable and also widely considered to have the lowest risk of bleeding out of all our P2Y12 inhibitor options.
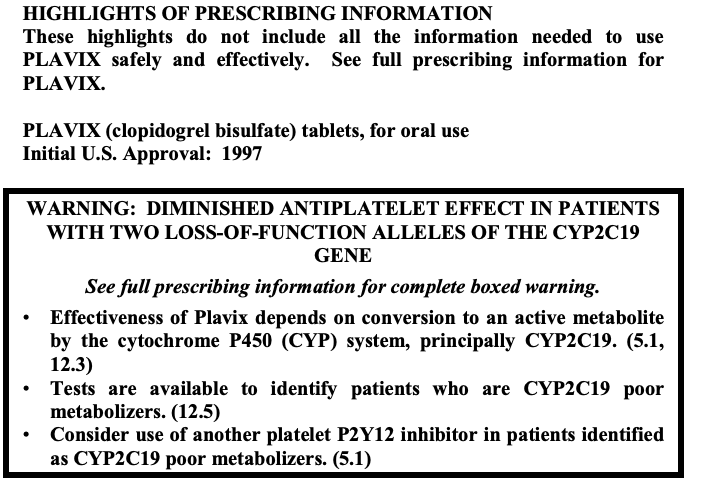
Cons: Clopidogrel is a prodrug which means she’s biologically inactive until she can be metabolized to produce an active component. Clopidogrel is metabolized by an enzyme known as CYP219 in the liver. Unfortunately, due to the wonderful world of genetics, different people can have different polymorphisms of CYP2C19 – some people may naturally have a less active version of CYP219 (known as a poor metabolizer), some people may have an hyperactive version (known as an ultra rapid metabolizer) and so on. Because the efficacy of clopidogrel is so reliable on its conversion from prodrug, that means some people (roughly 2-14% of the population) you give clopidogrel to may have reduced antiplatelet responses from clopidogrel (in practice we call these people clopidogrel nonresponders). Because of this, the FDA put a boxed warning in March of 2010 warning that this is a possibility. In addition, any drug that modulates the activity of CYP2C19 may also mess with the efficacy of clopidogrel so, when in doubt, run a quick drug-drug interaction checker on your patients.
Just like aspirin (ASA), clopidogrel should be given as a load (usually 300-600 mg), followed by a maintenance dose of 75 mg PO (by mouth) QD (daily).
Next on the block came prasugrel. FDA approved in 2009 thanks to the TRITON-TIMI 38 trial, it was found that – when compared with ASA + clopidogrel, prasugrel + ASA reduced the composite endpoint of CV morbidity and mortality while having an increased risk of bleeding.
Because of this increased risk of bleeding, prasugrel is contraindicated in patients >75 years old or in those who have a history of stroke or transient ischemic attack (TIA).
Just like all the above antiplatelets in ACS, prasugrel should be given as a load of 60 mg PO followed by a maintenance dose of 10 mg PO QD or 5 mg PO QD for those < 60 kilograms.
Now available as a generic, prasugrel was historically considered as having the highest risk of bleeding of the oral P2Y12 antagonists, though the recent ISAR REACT 5 trial may disagree.
The last oral P2Y12 inhibitor, ticagrelor, was FDA approved in 2011 with the PLATO trial. The PLATO trial was an international trial and, interestingly enough, the authors found regional differences with poorer outcomes in North American patients. Though the regional variations could have been by chance alone, investigators noted that, at the time of its publishing, patients in North America were on high dose (>300 mg) aspirin at much higher rate than the rest of the world (53.6% vs. 1.7%). Europeans at the time were taking 100 mg PO QD (our closest USA tab would be 81 mg). Because of this, per FDA labeling, the max dose of ASA that can be taken concomitantly with ticagrelor became the European 100 mg dose.
Because of the higher risk of bleeding seen with ticagrelor versus clopidogrel in the PLATO trial, ticagrelor is also contraindicated in patients with a history of intracranial hemorrhage (brain bleeding).
Ticagrelor is the only oral P2Y12 inhibitor that is dosed twice a day (BID) and also possesses the unwanted side effect of dyspnea (difficulty breathing), thought to be due to adenosine, since ticagrelor inhibits its clearance and increases its concentration in the bloodstream but it’s actually not completely elucidated. Like all above, ticagrelor should be given as a load of 180 mg followed by a maintenance dose of 90 mg PO BID.
Less commonly seen and used, cangrelor (Brand name Kengreal) was FDA approved in 2015 and is our only IV P2Y12 inhibitor. I won’t get too far into the weeds, but cangrelor is a good option if your patient is unable to take PO due to nausea/vomiting/unconsciousness. After all, if you patient can’t take anything orally, they can’t get their aspirin or their oral P2Y12 inhibitors and their platelet pathway is still fully active. Big thing to remember with cangrelor is because of its quick-on, quick-off properties, you still need to load your oral antiplatelet therapies as usual once its turned off.
Anticoagulation
Now that we’ve nailed down DAPT which will interfere with your platelet pathway, let’s talk about how we interfere with the other side of forming a thrombus – or the coagulation cascade.
Anticoagulation is recommend for ALL patients and is generally continued for at least 48 hours or until patients go to PCI (we’ll discuss PCI in a later post).
All of our anticoagulants interfere somewhere within our coagulation cascade to prevent further clot from forming. This is a big distinction between giving anticoagulants or giving clot-busting drugs such as fibrinolytics. Anticoagulants do nothing to existing clot, but they prevent that clot from getting bigger.
Your choice of anticoagulants in ACS tend to be given parenterally and include the following: unfractionated heparin (UFH), enoxaparin, bivalirudin, and fondaparinux. Believe it or not, but (to my knowledge) there’s actually no trial supporting the use of UFH in ACS since it was at one point the only thing we had.
See below for dosing strategies and supporting evidence. I eventually plan on doing a future post discussing the basics of anticoagulants and how each agent works.
| Agent | Dosing | Trial |
| UFH | 60 units/kg bolus IV 12 units/kg/hr IV infusion | — |
| Enoxaparin | Optional 30 mg IV bolus 1 mg/kg subcut BID; (can consider 0.75 mg/kg subcut BID in patients >75 YO) | ESSENCE SYNERGY |
| Bivalirudin | Prior to PCI: 0.1 mg/kg IV bolus then 0.25 mg/kg/hr IV infusion At the time of PCI: 0.75 mg/kg IV bolus, then 1.75 mg/kg/hr IV infusion | ACUITY |
| Fondaparinux | 2.5 mg subcut daily Do not use alone for PCI secondary to increased risk of catheter thrombosis | OASIS-5 and OASIS-6 |
The “MON” of MONA (and other things)
That we’ve went over the key meds that actually will treat the underlying cause, let’s talk about other meds you might give in initial ED management. Let’s start with the “M” in MONA: morphine.

Morphine is an opioid medication that comes from the poppy flower and is used to treat pain. Besides its analgesic effects, it also has anxiolytic and venodilatory effects.
The problem I have with morphine (and MONA), is that morphine shouldn’t be a staple of use in your ACS patients. A side effect of opioids is that they delay gastric emptying – in other words – they slow the rate at which your stomach pushes its contents into your intestines. This becomes a problem since all of your important oral meds (ASA, P2Y12 inhibitors) are usually given orally and are absorbed in the intestines. The hypothesis that morphine may delay the effects of your antiplatelet agents/worsen outcomes was actually proven in a recent 2020 study.
OK, OK – so should you not treat pain in your patients? Well, that’s a bad idea too. Think about what patients look like when they are in pain – think about their vitals. They’re hyperventilating and their heart rates are elevated (tachycardic).
Now consider what effect that would have on their heart’s demand. It would increase its demand right? The problem is, thanks to that pesky thrombus, our supply is limited or blocked and your heart will be unable to get enough blood to supply it, which would then worsen necrosis and cardiac cell death.

TLDR: if your patient is in pain, give them the damn morphine (or fentanyl) if a nitrate isn’t helping. But if they’re hanging in there – it might be better to hold off.
Next is oxygen – let’s make this simple. This shouldn’t be a staple either – if you patient needs oxygen – let’s say they have an O2 sat <90% – slap on a nasal cannula and start that baby at 2-4 L/min. If they don’t – hold off.
Next up are nitrates like nitroglycerin (NTG). NTG can be given sublingually, as a spray, put as a paste on the chest, or given as a continuous infusion (CIVI).
Nitroglycerin works by converting to nitric oxide in the body which causes relaxation of smooth muscle within blood vessels and causes vasodilation. This can help get more blood flow through the clogged coronary artery and therefore help with chest pain.
Nitroglycerin tends to work primarily on preload (by dilating peripheral veins) but at higher doses, starts dilating peripheral arteries at well, thus at these higher doses you can see both preload and afterload reduction.
The main things to know about NTG in ACS:

- Contraindicated in patients who have taken a PDE-5 inhibitor within 24-48 hours. Examples of these drugs include sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra) and avanafil (Stendra). Remember that PDE-5 inhibitors are not only used for erectile dysfunction – so don’t forget to ask your female patients about this use as well! The reason why this combination is contraindicated is because PDE5 decrease the metabolism of nitric oxide induced activation of cGMP, so they allow even more smooth muscle vasodilation. This combination could potentially cause a massive, and potentially even fatal drop, in blood pressure.
- Avoid in patients that already have hypotension.
- Use with extreme caution in patients that have a right ventricular infarct. Why? Well, going back to our coronary anatomy talk, remember that the RV is not designed to pump blood through systemic circulation – it only has to pump blood through the pulmonary circulation. The pulmonary pressure is way less than the systemic pressure (think of a pressure between 8-20 mmHg versus like 80 mmHg). The right ventricle is heavily dependent on preload, especially during diastole, because veins do not muscular walls to keep blood moving like the arteries. The right side of the heart doesn’t really create much suction from contractions to pull that blood in. Because of this, reductions in venous return – or preload – will result in less pumping pressure of the RV which will lead to lower pulmonary circulation, lower LV filling, lower CO, lower BP and then really bad things could happen.
That’s the basics of ED management. Still to come: reperfusion strategies and meds to initiate while in-house or on discharge. Until then –

You’re so awesome! I don’t suppose I have read something like this before. So great to discover someone with some original thoughts on this issue. Seriously.. many thanks for starting this up. This site is one thing that is required on the internet, someone with some originality!
LikeLike
There is definately a great deal to know about this topic. I love all the points you have made.
LikeLike