Today we’re going to be talking about reperfusion strategies. Reperfusion is defined as restoration of blood flow to a previously ischemic organ or tissue – and in the case of ACS (and this blog) we are talking about the heart.
Let’s start with STEMIs. As discussed before, STEMIs are the worst type of MIs and often represent a complete occlusion of coronary artery blood flow. In the case STEMIs, remember that time is heart.

STEMIs
Patients with STEMIs need revascularization ASAP – and all eligible STEMI patients should go to PCI if symptoms onset has occurred within the last 12 hours.
Keep in mind that not every hospital has a cath lab. If your patient arrives at a PCI-capable hospital, the ideal first medical contact-to-device time is less than or equal to 90 minutes. If your patient happens to arrive at a non-PCI capable hospital, the ideal first medical-contact to device time (AKA transfer to a PCI capable hospital) within 2 hours.
But what the heck is PCI?
PCI stands for percutaneous coronary intervention. PCI refers to a group of minimally invasive procedures used to open those clogged coronary arteries.
In PCI, the interventional cardiologist will insert a catheter (a thin flexible tube) usually through either the femoral or radial artery. The doc will puncture either the femoral artery in the leg or radial artery in the arm with a needle and pass a wire into the artery. They will then thread this catheter all the way up to the heart and place the tip of the catheter right at the mouth of the coronary artery.
The doc will then inject radio-opaque dye (usually iodine-based) through the catheter. By using radio-opaque dyes, X-ray can then be utilized to literally visualize the vasculature of the heart.


After visualizing the area of infarct, the interventionalist will determine if they just want to open the area up with a balloon catheter (aka balloon angioplasty) or if they also want to put in a stent.

The balloon angioplasty will push all the area of infarct open and the stent (a metal structure inserted in the vessel) will help to keep the area open.

Thinking about our coagulation cascade, keep in mind that the introduction of this foreign catheter into your vessels will trigger thrombus formation in and of itself. That is why anticoagulants (either UFH or bivalirudin) should be onboard during PCI itself.
A GP2B3A inhibitor (e.g. abciximab, tirofiban, or eptifibatide) is an IV anti-platelet agent that can also be given during PCI, though it is not universally used. GP2B3As are generally used at the discretion of the interventionalist, depending on how much clot burden they are seeing.
GP2B3A inhibitors work to inhibit the GP2B3A receptors found on platelets. GP2B3A receptors bind to fibrinogen (aka factor I) resulting in the ability of platelets to stick together (since they can attach to the same strands of fibrinogen, resulting in a clot).
Because the GP2B3A inhibitors increase the chance of bleeding (and a lot of data supporting their efficacy was in the time prior to us having the potent P2Y12 antagonists), they aren’t routinely added during PCI, they can be considered in the follow circumstances:
- Your patient has high risk features
- Your patient has a large thrombus burden
- Your patient was not adequately loaded with a P2Y12 inhibitor (although these days, we now have cangrelor for this)
Depending on your patient’s specific coronary artery anatomy, location, or # of vessels diseased, your patient may or may not be candidates for coronary artery bypass grafting, or CABG.

CABG is an invasive (and I mean open-chest, open up your ribs type of thing) surgery that will restore blood flow to an obstructed coronary artery. The doc will actually use a vessel from the patient’s own body as a graft to connect to the area distal to the blocked area of the coronary artery.
There’s two main approaches in CABG to vessels. The first is using the left internal mammary artery (LIMA) – in this procedure, the surgeon will divert the LIMA to the left coronary artery. This method differs from the second method because the artery still is anchored in its original spot.

The other approach involves taking a great saphenous vein completely out of the leg. One end is attached to the aorta and the other attached to the coronary artery after the obstruction.
The procedure is often done with the heart stopped using cardiopulmonary bypass (CPB circulates and oxygenates blood for the body while bypassing the heart and lungs) – but doesn’t have to be. The other alternative is “off-pump” beating heart surgery.
You may also hear terms like “double bypass” versus “triple bypass” which refers to the # of coronary arteries that are bypassed.
The problem we run into a lot when the interventionalist opts to consult the cardiothoracic surgeon is that our patient was just loaded with potent antiplatelet agents (specifically the P2Y12 inhibitors) that make performing an open chest procedure too high risk of bleeding.
The recommended duration of holding your antiplatelet agents are as follows:
| Antiplatelet Agent | Recommended Duration to Hold Prior to CABG |
| Prasugrel (P2Y12 inhibitor) | 7 days |
| Clopidogrel or ticagrelor (P2Y12 inhibitors) | 5 days |
| Abciximab (GP2B3A inhibitor) | 12 hours |
| Eptifibatide or Tirofiban (GP2B3A inhibitors) | 2-4 hours |
| Aspirin | 81 mg is OK to be continued throughout surgery |
We also have fancy platelet function tests and platelet mapping that can tell you if you can do the surgery any earlier than this. Because this waiting period, it’s not uncommon to have our ACS patients sit in the hospital for days waiting for their P2Y12s to wash out of their system as they await CABG.
NSTEMIs and UA (Collectively, NSTE-ACS)
You have to keep in mind that going into the cath lab, though done routinely, doesn’t come risk-free. And yes – with STEMIs – the benefits of getting PCI outweigh those risks. But what about in our NSTE-ACS patients?

There are actually two main pathways that we go down with our NSTE-ACS patients.
- Ischemia Guided (AKA Medical Management) Pathway
- Early Invasive Pathway
The first thing to do when your patient comes in with NSTE-ACS is determine if they are high-risk enough to get into that cath lab ASAP.
In order to stratify these patients, we use risk calculators such as the TIMI UA/NSTEMI to figure out what these patients’ risk at 14 days is for all-cause mortality, new or recurrent MI, or severe recurrent ischemia requiring urgent revascularization.
For NSTE-ACS patients with high enough prognostic risk, they will follow the early invasive pathway, which means the patient should go to the cath lab and get a diagnostic cardiac cath within 24-72 hours and figure out if your patient needs PCI vs CABG.
All other NSTE-ACS patients will start off with the ischemia guided pathway. The ischemic guided pathway is also called med management and basically means you should treat your patient with all the good ACS ED management stuff but hold off going to cath.
Sometimes my learners will forget that ischemia guided means med management so my advice is to think about the words – “ischemia guided” – aka let the patient’s degree of ischemia guide you to figure out if they are OK just with meds or if it is telling you the patient should get to the cath lab.
Once you stabilize your NSTE-ACS patients with medical therapy, and they’re not getting any better, cath could still be an option. Which is why in the ischemia guided pathway, we reserve invasive eval (aka cath) only for patients:
- Failing medical therapy
- Having evidence of ischemia with non-invasive testing (e.g. troponins keep rising, etc)
- Who had high prognostic TIMI (or other tests like GRACE) scores
Stents and the risks that come with them
Now that we talked about the basics of reperfusion strategies and the difference between our strategies for STE-ACS versus NSTE-ACS, let’s talk a little bit about the types of stents our patients can get.

The two major categories of stents are bare metal stents (BMS) and drug eluting stents (DES).
Now to really get a good idea about how these stents differ, you have to have a basic understanding of what is happening in the body when a stent is inserted.
When you have a stent inserted into your coronary artery, I want you to immediate think back to the clotting cascade, which can be triggered by the presence of foreign objects in our bloodstream. Enter stents.
As long as that stent material is exposed to your bloodstream, you will run the risk of getting in-stent thrombosis and get a clot within that new stent.
But when you get a stent inserted, it doesn’t stay exposed to your bloodstream forever. Your body actually will start endothelializing that stent and eventually incorporating it into the vascular wall. And so, if we were in that coronary artery years after that stent was placed, we wouldn’t be able to physically see it anymore – it would be underneath your vessel wall.
The problem is – your body does its best, but it’s not perfect. And when it grows over that stent, it doesn’t do so neatly. In fact, it can often overgrow over the stent surface – so much so that the newly formed tissue will start blocking some of the coronary artery flow again. This is known as stent restenosis (remember that the word stenosis means a narrowing).
Now that we got that down, now we can talk about the differences between the stents. And the key to remembering the difference is knowing what kind of drugs are actually in the drug eluting stents.
The meds that are infused within these stents are meds like sirolimus or everolimus. These meds are anti-proliferative agents. These meds inhibit cellular growth. But because these meds slow the endothelialization process, that stent takes longer to get hidden away into the vessel wall, and stays exposed to the bloodstream for longer.
Bare metal stents are what they sound like – bare metal. They don’t have any drugs infused into them. And so because of this, your body will be able to cover the stent structure up sooner, since your cell growth will proceed as normal.
Question: When comparing DMS versus BMS, compare their relative risks of –
- Restenosis
- Thrombosis
I’ll give you a second.

If you said BMS have a lower risk of thrombosis but a higher risk of restenosis you would be right. Conversely, DES have a higher risk of thrombosis but a lower risk of restenosis.
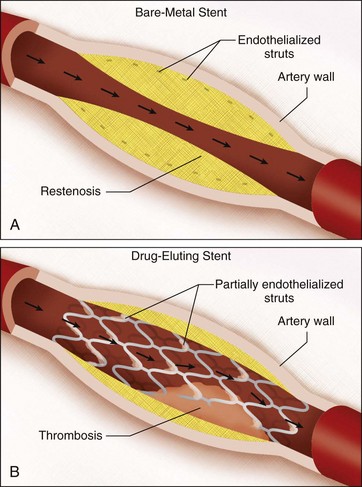
Why does it matter? Well, when patients get stents, dual-antiplatelet therapy (ASA + a P2Y12 inhibitor) is key to prevent thrombosis within that newly inserted stent. I can’t stress how important it is.
But, what if your patient has a history of really poor compliance and doesn’t take their meds? Well, in cases like this, it may be better to give these patients a BMS since the risk of thrombosis will decrease faster than it would if they got a DES.
Fibrinolytics
I’m not going to go into the weeds with lytics, but lytics are in the guidelines, and may be your patient’s only option depending on what hospital you are in.
The main difference between anticoagulants and fibrinolytics are that anticoagulants only prevent further clot from forming, whereas lytics actually break down existing clot.
We didn’t mention this during our coag cascade talk, but just like most other things in the body, your body has different ways of maintaining homeostasis in the body. In other words, your body has a system of checks and balances to keep processes in check.

The plasminogen activator/plasmin system is a cascade your body has to control fibrin degradation and promote the breakdown of clots. When plasminogen turns into plasmin, plasmin can breakdown fibrin and therefore break down the clot.
Commonly used lytic meds like tenecteplase and alteplase promote the initiation of fibrinolysis by binding to fibrin and convert plasminogen to plasmin which accelerates the breakdown of clots.
The problem is that lytics are very high risk medications. If you think anticoagulants have a high risk of bleeding associated with them, wait until you meet lytics. And if you check out Lexicomp, check out the long list of contraindications and relative contraindications for the lytic agents. If you are in the situation where a lytic is given – always pull up this list and go through each criteria.
The main thing I want you to remember about lytics is that because they are such high risk meds, unless your patient has a STEMI, they are not candidates to get a lytic. In other words, NSTE-ACS is just not severe enough to outweigh the risks seen with administering lytics. Only the most severe type of ACS – STEMIs- would be when you would consider them.
And lytics should still not be considered first line for our STEMI patients. Remember when we said we want to get your STEMI patients to a PCI-capable hospital within 2 hours? Well, if you are at a rural community hospital and there are no PCI capable hospitals within a 2 hour radius, now is the time to use lytics.
Remember – no STEMI, no lytics.

Please, please, please do not forget:
If using lytics for STEMI, do not forget to start anticoagulation. Why?
Let’s think about it. Your patient has a huge clot in their coronary artery because their vessel wall has busted open due to plaque rupture. You give them a lytic, which will dissolve existing clot. Problem solved, right?
Not exactly. Even though you broke down existing clot, you didn’t ✨magically✨ fix the damage to the vessel wall. Which means that tissue factor will still be secreted out, which means…..you guessed it, more clot will want to form.
You need to give an anticoagulant to prevent further clot from forming at the site.
Anticoagulants should be given until revascularization is performed (e.g. PCI) – if that’s not feasible, they should be given for at least 48 hours or for the duration of the hospital stay up to 8 days. UFH or LMWH can be used.
Next post we will be finishing up our ACS talk with the meds we want to send our patients home on –

One thought on “ACS Part 4️⃣: Reperfusion Strategies”