Hey everyone. Today I wanted to talk in a very basic way about how clots are formed and clarify a little misconception between the terms “antithrombotics”, “anticoagulants”, “thrombolytics”, and “antiplatelets”.
CARDS often goes hand-in-hand with the use of all the above classes of meds (^^^) and before we go ahead and delve into them, I want you to get a basic understanding of how clots are actually formed, so then you can better appreciate the action of those drug agents later on.
Clot formation is actually pretty complicated, with a ton of different biochemical processes happening simultaneously, but for today, let’s focus on the big picture.
There are two main pathways that are involved in creating a clot: the platelet pathway and the coagulation cascade.
Both of these are triggered during clot formation, and both play an integral role in the final clot that is made. Let’s start with examining the platelets.
Platelets are what we call these teeeeeny anucleate (without a nucleus) cells that are disk-shaped.
These little guys end up being key in blood clotting. The average person has a trillion platelets, and platelets turnover (die and are replaced) about every 10 days.
Side note: there’s actually a huge shortage of blood rn in the United States. The biggest we’ve seen in years. Please consider donating or encouraging others to donate! Click >>here<< to see drives near you! I recently got my squeamish husband to donate. If he can get through it, there’s hope for you too.

Your body is really smart. At baseline, your platelets circulate through the body in this inactive, quiescent state. They keep calm, carry on – they don’t want to initiate blood clotting in the wrong place. After all, blood clotting – though it can save lives when needed – can also be fatal in and of itself (e.g. pulmonary embolisms, strokes, etc).
These inactive platelets are disk shaped (like the photo above), due to a microtubule ring around the edge of the cell. The platelets are also decked out in a bunch of surface proteins which interact with things like the vessel wall, other cells, substance in plasma, etc and will later play a big role.
Inside these platelet cells are a bunch of little sacks – or granules – that contain a bunch of different proteins and chemicals. These things will be super important when the platelet becomes activated, but at baseline, the platelet just chills out and keeps all this stuff tucked away inside.
! Damage Occurs !
Let’s say you have a site of injury and the body wants to do its thing to prevent a lot of bleeding in that area.
In a healthy blood vessel, the internal wall of that blood vessel is intact and smooth.
When there is damage to the wall (like in that GIF above), that break in the vessel wall will expose proteins called collagen and Von Willebrand factor. These proteins are normally located inside the vessel wall – but when that wall breaks open – they are exposed to the bloodstream.
Platelets will start the blood clotting process by tethering to and literally rolling around onto damaged endothelial cells at the site of injury. This is all thanks to the now exposed collagen and Von Willebrand factor (vWF). vWF will bi
The platelets have a receptor located on their surface called glycoprotein Ib-IX-V (aka GPIb-IX-V….a terrible name I agree). The vWF will bind the platelets onto the endothelial surface by binding to the GPIb-IX-V receptor on the surface of the platelets. This will cause the platelet to be pulled out of the circulation and literally roll and tether onto the surface of the damaged endothelium, forming a monolayer of adhesive platelets.
Once these platelets bind to the site of injury, they become activated, and change shape. They get all cool looking and wonky.
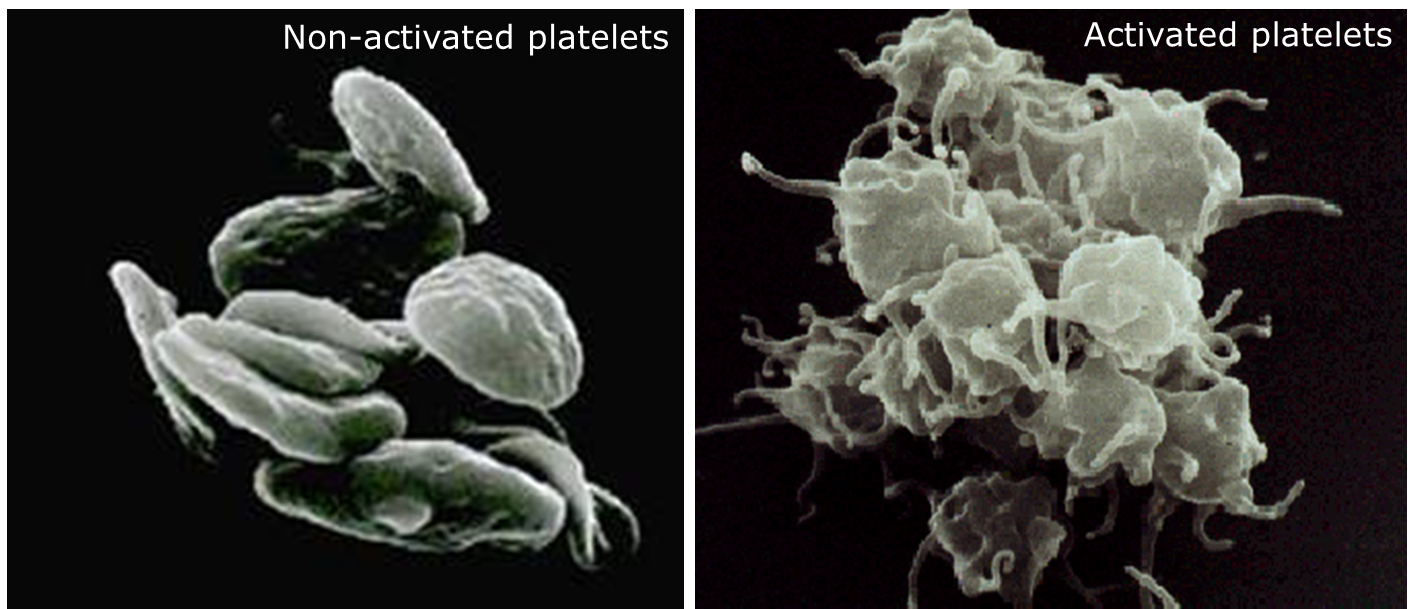
Once it changes from its quiet state to its active, dendritic form, the platelets will start releasing their inner contents – prothrombotic molecules like ADP and thromboxane A2.
ADP by itself is not that great at stimulating the secretion of further granule contents in platelets. However, its interaction with a receptor known as P2Y12 can accelerate the secretion of platelet granules. P2Y12 also plays a big role in the stabilization of platelet aggregates.
ADP binds to its receptors and will induce aggregation and help to recruit further platelets to the site of injury.
Besides ADP, proteins called thromboxane A2 will also be spit out from the platelets. Thromboxane is integral because it stimulates the platelets to start cross-linking with each other.
The release of ADP and thromboxane stimulates a cascade that will cause the association of proteins called gpIIb and gpIIIa on the surface of the platelets. This activated GPIIbIIIa complex is capable of binding to factor I (aka fibrinogen) which is a long stringy protein. As more platelets activate GPIIbIIIa, platelets will aggregate or “stick together” as multiple platelets can stick the same strand of fibrinogen. When ADP binds to the P2Y12 receptor, it will also cause an increase in affinity of these GPIIb/IIIa receptors.
With all these proteins being spit out from the platelets, the clot will start to grow rapidly at the site of injury.

So to review – the platelets are pretty neat. They wait until they are activated, change shape, spit out their contents, and then recruit each other and cross link. The whole purpose of the platelets is to create that nice platelet plug at the surface of injury to help prevent any further bleeding. Let’s say it again:
The main purpose of platelets is to form a nice platelet plug at the site of injury to prevent further bleeding.
An agent that works on the platelet pathway above is known as an antiplatelet agent.
Now let’s move on to the coagulation cascade.
We just discussed that the point of the platelet pathway is mostly to create a plug at the site of injury right? Well have you ever considered what’s the whole point of the coagulation cascade?
To think this through, let’s see what the end product of the coagulation cascade is.
Cue the diagram that sends students everywhere into panic and dread.

If you look at the above diagram, you’ll see that the end product is something called fibrin. Let’s say it again:
The main purpose of the coagulation cascade is to produce fibrin.
But what is fibrin? Well, I like to think of fibrin as a mesh net. The purpose of this mesh net is to grow around the existing platelet plug to solidify that platelet plug.
Check out the visual below. Through a series of enzymatic steps, the clotting cascade ends up producing fibrin.
Now – when talking about the coagulation cascade, there are two main things that can trigger the production of fibrin in the blood – what we call the intrinsic pathway and the extrinsic pathway.
These are just fancy names for things that will stimulate the coag cascade. The extrinsic pathway is triggered by actual vessel injury. A protein called tissue factor is normally present in the endothelial lining (aka the inside of the wall of vessels).
Without injury, that tissue factor (TF) will be contained within the vessel wall. However, when the vessel wall is damaged, tissue factor will be exposed and trigger the activation of factor VIIa which will then cause a series of reactions to ultimately produce fibrin.
The intrinsic pathway is triggered by the presence of foreign objects within the blood. If your blood comes into contact with an something foreign, the intrinsic pathway will be activated, causing a series of reactions to, you guessed it, ultimately produce fibrin.
I tend to remember the cause of these pathways by thinking that the extrinsic pathway is caused by external damage whereas the intrinsic pathway is caused by something present in the blood. Not sure if that will help anyone but it’s worth a shot 🤷🤷.
The clotting cascade can be triggered by one of two pathways: the intrinsic cascade (caused by vessel damage) and the extrinsic cascade (caused by contact with a foreign surface).
The common pathway is so aptly named because it is shared by both pathways. In other words, no matter what triggers the initial reaction/stimulus, you will end up stimulating factors X, V, II, and I.
The trick way I remember the factors of the common pathway is thinking about dollar bills. In the US, we have a $10 bill, $5 bill, technically have a $2 bill and a $1 bill. Those are the same factors in the common pathway (e.g. we don’t have an $11 bill or $9 bill, etc).

Protip: To remember the factors of the common pathway, think about dollar bills.
Another note: the majority of clotting factors are synthesized in the liver. This is why patients with liver dysfunction may present with elevated INRs, or have issues with bleeding.
Any agent that acts to prevent the formation of or inhibit any of the clotting factors is known as an anticoagulant.
Keep in mind that an anticoagulant will do nothing to any existing clot. It will only prevent the formation of further clot, or in other words, prevent that clot from getting larger.
Next: what is an antithrombotic agent?
Well, because a thrombus is another term for a clot, an antithrombotic agents emcompasses any agent that prevents clot formation.
An antithrombotic agent is anything that prevents formation of a clot (this includes both antiplatelet agents and anticoagulants).
Lastly, like I said before, this process is a lot more complicated than the above. The platelet cascade ends up stimulating the coagulation cascade as well and the coag cascade occurs on the phospholipid surface of the platelets.
Thrombolytics
Just like many, many other processes in your body, your body has a “checks and balances” system to keep things in check. We already reviewed how your body forms a clot. But what does it do to help break down a clot or get rid of a clot?
This is where a protein known as tissue plasminogen activator, or tPA comes into play. tPA is an enzyme that converts plasminogen to plasmin.
Plasmin is responsible for breaking up any existing clot and degrading it. So when we give anticoagulation to patients with a new deep vein thrombosis (DVT), or a pulmonary embolism (PE), etc, we are preventing more clot from forming and allowing our body to do its thing to break down the clot naturally/on its own through plasmin.
Thrombolytic agents, unlike anticoagulants, break down existing clot.
In practice, we have agents (included IV tPA) that we can give patients to cause this process to happen. These agents carry a very high risk of bleeding with them, but can also be lifesaving if used in the correct circumstances.
That is thrombus formation in a very, very 1,000 foot view. Hopefully, although we stayed pretty basic, you can better understand future discussions on antithrombotic and lytic therapies.
See ya later!





I couldn’t refrain from commenting. Very well written!
LikeLike