Welcome back to the blog 👋👋👋. Today we’re going to discuss a classic med that, despite decades of advancement in medicine, probably isn’t going anywhere anytime soon – warfarin. If you’ve followed this blog for a bit, you’ll know that I’m kinda into history and learning about where stuff comes from. Let’s get into it.
Fun fact: warfarin was once used as a rat poison. But in fairness, to quote Paracelsus, “the dose makes the poison” of about anything in life…..(I’m looking @ u, cheese. I’m lactose indenial).

The History of Warfarin
The year is 1920. You are a cattle farmer hanging out on the prairies of Canada/North America when, you start noticing something going on with your livestock. A bunch of your previously healthy cattle start to die – they all appear to been suffering from internal bleeding, but with no obvious cause.

You touch base with your farmer neighbor a few miles down the road – and they have the same thing happening with their livestock. You guys talk and figure out that this tended to happen most commonly when the climate was damp.
You try to brainstorm a reason – was it trauma? An infection? Has there been anything new or different in the cattle’s routine?
You realize their feeding routine has been a little different. Due to some financial hardship, you’ve had to keep their old sweet clover hay that they eat in their bales – normally you would have thrown in out when it got a little moldy, but with the current financial market, you just couldn’t afford to replace their food so often like you normally would. You also notice that the sweet clover hay they eat tended to get even moldier when the weather was damp, which is when the cattle tended to do the worst.

This seemingly idiopathic (aka we don’t know the cause) bleeding was coined to be known as “sweet clover disease“.
Farmers at the time found out that a few things seem to help the livestock:
- Giving the cattle fresh blood/transfusions
- Getting rid of the moldy hay

It wasn’t until the 1940s, when they figured out that there was this natural substance called coumarin in the sweet clover that was oxidized when it became moldy and turned into dicoumarol. This compound was found the be the culprit, and the reason these cows were bleeding. This dicoumarol was the basis for the compound we now know today as warfarin.
This is how warfarin was discovered. But they also figured out pretty early on that there were varying degrees of responses and that the degree of anticoagulation had to monitored by laboratory methods – this is where the INR came into play (if you need a refresh about the INR, check out this post.
Warfarin’s Mechanism of Action
Warfarin is a super unique drug. Unlike other anticoagulants, warfarin does nothing to existing clotting factors.

👏Let’s👏 say 👏it 👏again👏:
Warfarin does nothing to existing clotting factors.
Unlike our other anticoagulant agents, which either directly or indirectly inhibit existing clotting factors, warfarin does nothing to existing clotting factors.
Remember that our clotting cascade is made up of multiple clotting factors that ultimately creates a fibrin clot.
If you need a refresh on how clots are formed, check out this post.

So – if warfarin doesn’t do anything to existing clotting factors, how does it work?
Let’s go to the liver!
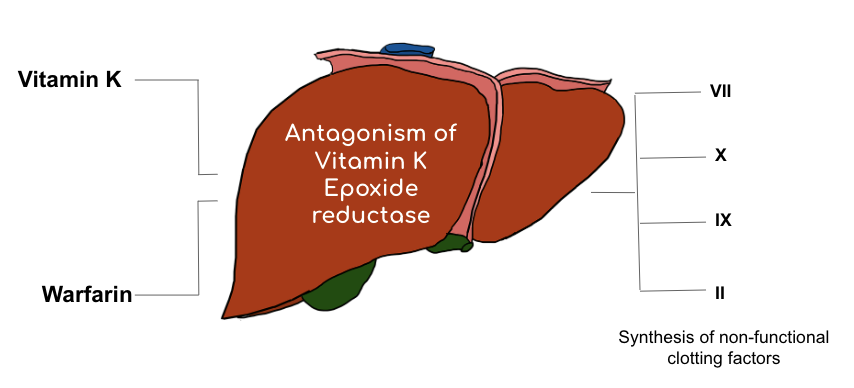
Your liver is responsible for making the majority of your coagulation factors.
Some (but not all) of your clotting factors require vitamin K to be synthesized. Without available vitamin K, these functional clotting factors can’t be produced.
Now, your body is pretty snazzy. She’s a resourceful queen. Once “used”, your body is able to “recycle” its stores of vitamin K to be used again.
The reduced form of vitamin K is the useful form – your body utilizes this reduced vitamin K as a cofactor in order to make certain clotting factors. But, in the process of making these active clotting factors, the reduced vitamin K becomes oxidized.
This oxidized form can’t do anything to create new clotting factors.
Luckily for us, our body has an enzyme called vitamin K oxide reductase (aka VKOR) that is able to take that useless oxidized vitamin K and convert it back into its helpful reduced vitamin K version.

So to reiterate – under normal circumstances – your liver makes these clotting factors by using vitamin K; in order to keep an active supply of vitamin K to facilitate the creation of these clotting factors, your body has this VKOR enzyme that “recycles” vitamin K in your body so the process can continue on, again and again.
This is where warfarin works.
You’ll sometimes see warfarin written as being a “vitamin K antagonist” – but more correctly, it is a VKOR inhibitor.
By inhibiting the VKOR enzyme, warfarin prevents your body from making vitamin-K dependent clotting factors.
So warfarin works by preventing NEW clotting factors from being created. But don’t forget that your body already has existing clotting factors that were made beforehand that are still floating around out there. These clotting factors that are already made are still going to have a thrombotic (pro-clotting) effect.

The specific clotting factors that warfarin inhibits the production of are factors VII (seven), IX (nine), X (ten), and II (two).
Now, if you need a trick to memorize these, I learned the “snot” pneumonic back when I was in school. Kinda gross, but it does the trick. The pneumonic is
Seven
Nine
1O
Two
The 10 is slightly tricky since the O makes up the “0” in “10” but hey, it helped me.
[Insert funny snotty gif here except that snot is like the one thing that really makes me dry heave so I personally just can’t handle that in my life rn]
Besides factors VII, X, IX, and II, warfarin also inhibits the creation of something called protein C and protein S.
Protein C and protein S are your body’s natural anticoagulants.
This will be important to keep in mind shortly!

Let’s recap.
We start a patient on warfarin.
Warfarin starts preventing the creation of new protein C, protein S, factors XII, X, IX and II. Your body still has previously created protein C/S, factors XII/X/IX/II floating around in your bloodstream.
Now we have to wait for those existing factors/proteins in your blood to degrade.
However, not every protein/clotting factor is going to degrade at the same rate. Each factor/protein is going to degrade at its own, individual rate. Each has their own, unique half life.
Keep in mind that half life is the amount of time it takes for 50% of a substance to degrade. The shorter the half life, the faster that substance will degrade.
Let’s use a silly, but practical example. The half life of metoprolol tartrate is ~3.5 hours. Let’s say you had 1000 molecules of metoprolol in your body after a dose. If we fast forwarded 3.5 hours from now, you would only have 500 molecules of metoprolol left.
Now – like I said before – each factor/protein will have their unique half life.

Check out the above diagram.
Do you notice anything??? Anything important?
I think there’s a couple of things to unpack here. First – remember that factor II (thrombin) is a big driver of thrombosis. We discussed this in our clotting post.
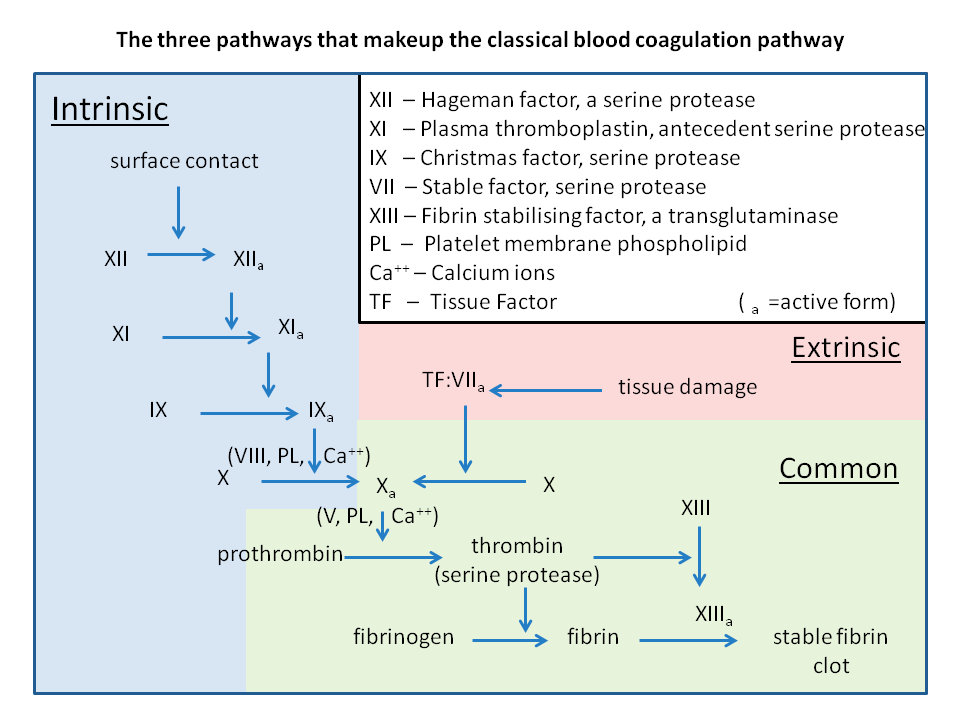
This should kinda make sense, since it’s just one step away from becoming fibrin.
If you look at the half life of factor II it’s a whopping 60 hours. That’s almost 3 days (!). This means that it’s going to take 60 hours for half of our existing thrombin to degrade out of our bloodstream and help get our anticoagulant effect of warfarin that we’re looking for. This means that warfarin really takes days to start working and having its full anticoagulant effect.
But also check out the half life of protein C.
8 hours.
And do you remember what we just said protein C’s role in the body is? It’s your body’s natural anticoagulant.
Now, this is kinda tricky because people’s brains hate double negatives but – if you inhibit these factors from being made, and your existing protein C drops first, and it’s your body’s natural anticoagulant….then what you’re actually going to end up with when you start warfarin is a prothrombotic effect. After-all, you still have high amounts of factors II, VII, IX and X floating around being able to exert their effects, but now you no longer have protein C to help keep them in check.
Based on the half lives of the proteins and factors warfarin inhibits, when you start warfarin, you actually are going to initially be pro-thrombotic.
This could be really troublesome in our high thrombotic risk patients right? Because not only should they be on an anticoagulant because they just had a DVT, or a PE, or whatever…but now they are actually more likely to clot than they were before starting their warfarin.

Bridging: What is it, Why do we Do it, and When?
Because of the above – warfarin depleting protein C before the other factors causing you to be hypercoaguable – sometimes we will opt to do something we call bridging.

Bridging is a term that refers to the use of quick-acting anticoagulants (typically heparin of low molecular weight heparins) for a period of time during the interruption or initiation of warfarin therapy when your INR is not yet within a therapeutic range.
In other words, we are “bridging” them with the anticoagulant effect that they need with a parenteral anticoagulant – one that will work in the moment – until the rest of those clotting factors degrade out and warfarin in and of itself has an anticoagulant effect in your patient.
We do this in high risk patients when their INR drops to a subtherapeutic range or when they start warfarin for the first time. You’ll dose them with warfarin, and start them on a therapeutic heparin drip, or give them therapeutic doses of enoxaparin. In other words, they will get both agents. Keep in mind this isn’t clinically the same as being on two therapeutic anticoagulants at the same time since we are waiting for warfarin to get to its full effect.
The general rule of thumb is that bridging should be continued for at least 5 days and after you’ve seen 2 therapeutic INR values.
Hopefully this time frame makes sense to you – after-all, keep in mind that factor II takes 60 hours to degrade by 50%. So even if you get premature changes in the INR that look therapeutic – it’s not clinically the same as a therapeutic INR of someone who’s been on warfarin for months since that patient still likely has that thrombotic factor II hanging around.
However, something to also keep in mind is that not every patient needs to be bridged. It all depends on their risks of clotting and their risks of bleeding.
When making or evaluating the decision to bridge, always consider the patient’s risk for clotting. Are they starting warfarin for atrial fibrillation? Did they just have a recent PE or DVT? Do they have a mechanical heart valve?
In general, anyone starting warfarin for a recent clot and/or mechanical heart valve should get bridged -because they are high risk of clotting at baseline and we don’t want to risk that pro-coagulant effect that warfarin has when starting.
However, if for some reason you have a patient with Afib starting warfarin – the risk is different. In non-valvular Afib (patients without a mechanical heart valve and/or moderate-severe mitral stenosis), we have a handy dandy risk calculator called the CHA2DS2-VASc Score. The higher your score, the higher the risk of thrombosis.
But just how thrombotic are these patients? Well, let’s look at it together.
*opens MDcalc* (link if you’d like to follow along: https://www.mdcalc.com/cha2ds2-vasc-score-atrial-fibrillation-stroke-risk)
Let’s give your hypothetical patient a CHA2DS2-VASc score of 3. What does the bottom tell you?

A CHA2DS2-VASC score of 3 gives you a stroke risk of ~3.2% per year. If you divide that by 365, you’ll find that this patients risk of stroke per day is about 0.008%.
Sure, that might sound like a little bit, but remember that atrial fibrillation is a chronic disease state. So sure, your risk per day might be low, but per year? Per decade? Chances are eventually these patients will be high risk of a developing a stroke in the long term (which is why we anti-coagulate these patients).
My point though – is that if we have an afib patient without any other comorbidities – I don’t really view this patient as being super thrombotic in the moment, and more often than not, we will decide not to bridge these patients.
The higher the CHA2DS2-VASC score, the more thrombotic their risk factors are – the more gray area this becomes. Always remember to look at the patient in front of you and assess them as a whole.
Knowledge check (yes this really happened to me).
You are practicing at a hospital. You get a call from a provider saying that he wants to start his patient on warfarin and wants them to be therapeutic tomorrow. He’s calling to ask about warfarin bolus dosing. How do you respond?
Hopefully, now that you understand just how warfarin works, you’ll tell the doc that warfarin doesn’t work that way. Sure, he could give an aggressive 30 mg dose, for example, but that 30 mg isn’t going to deplete your patient’s clotting factors by tomorrow – and chances are, your patient will just be supratherapeutic 3-5 days from now from that 30 mg bolus. All harm, no benefit. It’s a no from me dog.

Drug Drug Interactions
Something to keep in mind is that warfarin has a lot of drug-drug interactions. When it doubt, do a DDI check on Lexicomp.
Something that’s good to know though is that there are two forms (aka isomers) of warfarin – there’s S-warfarin and R-warfarin.
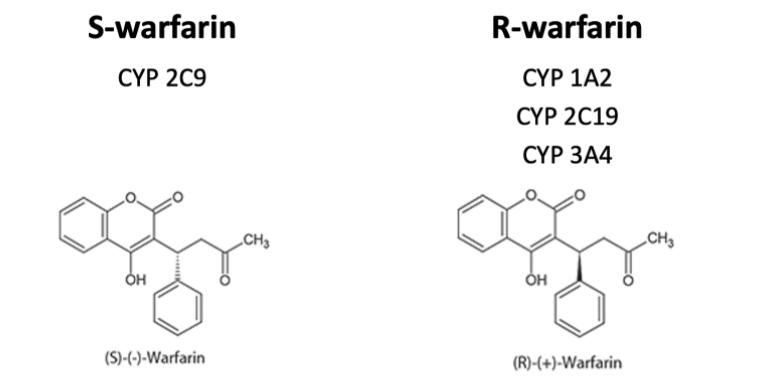
These different isomers are metabolized differently; S-warfarin by CYP 2C9, and R-warfarin by CYP1A2, CYP2C19, and CYP3A4.
However, the S-isomer version of warfarin has the majority of the anticoagulant effect.
Why does it matter?
As a rule of thumb, drugs that interact with the metabolism of S-warfarin (aka effect CYP2C9) usually are bigger deals – they often require preemptive changes in warfarin doses. I remember this by thinking about S- as the Super-warfarin. It’s the isomer that has most of the anticoagulant effect.
Drugs that effect CYP1A2, 2C19, and 3A4 tend to have less of an effect – you still care about these DDIs, but they might be managed just by simply monitoring and changing warfarin doses as you go.
Food Interactions
Warfarin also has pesky food interactions – anything that changes absorption of warfarin and/or vitamin K levels in your blood can effect response to warfarin.
Patients don’t have to eliminate vitamin K from their diets – they just have to try to stay consistent with their high vitamin K food intake – things like our leafy dark greens.

If you have a patient in the hospital (inpatient) you want to be careful of things like nutritional status, use of tube feedings, diarrhea and other things. All these factors can influence sensitivity to warfarin.
Drug/Disease Interactions
Another consideration is that disease states can influence sensitivity to warfarin. Let’s take a chronic heart failure patient on warfarin – oftentimes if they come with an acute exacerbation of their heart failure, they might be supratherapeutic on their INR labs. Oftentimes, this increase in INR is due to their acute illness rather than the dose itself. Be careful about making changes to these patients’ warfarin doses at discharge – chances are those doses that caused them to be supra-therapeutic on admission might be appropriate to keep them therapeutic when these patients are healthy.
Reversal
This is a whole other discussion, but the 10,000 foot view is that we can give patients vitamin K to reverse their anticoagulation from warfarin. We can also give them concentrated clotting factors II, XII, IX and II (drugs like KCentra, FEIBA) or give them clotting factors with blood products like fresh frozen plasma (FFP).
Dosing
Every patient will require a different dose of warfarin to keep them therapeutic. Common starting doses range from 2.5-7.5 mg.
Keep in mind that today’s INR is often not a reflection of the dose of warfarin the patient received last night – often it is a representation of the dose 2-3 days ago.
Warfarin is a narrow therapeutic index drug, meaning it has to be kept at certain concentrations to avoid treatment failure and toxicity.
Warfarin is color coded based on strength. If you are having a tough time figuring out a patient’s home dose, you can always ask them what color their tablets are.
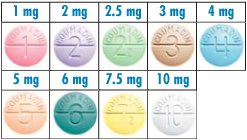
Indications
With the advent of the direct oral anticoagulants (DOACS) that tend to have less bleeding, warfarin’s use has decreased over the last few decades.
However, it doesn’t look like warfarin is going anywhere anytime soon. It’s still standard of care for disease states where we don’t have a lot of data with the use of DOACs and is also preferred over DOACs in certain conditions where the DOACs are contraindicated like in LVADs, mechanical heart valves, valvular atrial fibrillation, etc.
The infamous “rat poison” drug still holds an integral role in patient care.

I think that’s enough for today. Hopefully now that you have a better understanding of the unique mechanism of action of warfarin, a lot of stuff will make better sense. CYA l8r.


I love hearing the history of drugs/drug names. Your blog post are so great for a student like myself. In addition to your plethora of info I learned recently that the warfarin name came from the farmers that discovered it, Wisconsin Alumni Research Foundation (warf) + the molecule that you mentioned, (coum)ARIN = warfarin
LikeLiked by 1 person